Introduction
Chlorinated polyvinyl chloride (CPVC) has become an important engineering thermoplastic due to its relatively low cost, high heat deflection temperature, chemical inertness and outstanding mechanical, dielectric, and flame and smoke properties. First commercialized in 1959 by Lubrizol Advanced Materials, Inc. (formerly BFGoodrich Performance Materials), it has proven over nearly five decades to be a viable piping alternative for a variety of industrial applications that require a high use temperature and excellent resistance to corrosive chemicals.
The purpose of this page is to present a more detailed analysis of the chemical resistance capabilities of CPVC. It will provide specifiers and installers with information about those applications in which CPVC performs the best, as well as those in which its use may be limited or not recommended at all.
Variables that can affect chemical resistance include chemical concentration, temperature, pressure, external stress and final product quality. Because the number of possible use conditions is so large, the final decision regarding material suitability must often be based on in-service testing and direct communications with the piping manufacturer.
Below, we address capabilities and limitations that will provide general guidelines for an end-use application.
CPVC: An Overview
At its most basic level, CPVC is a PVC homopolymer that has been subjected to a chlorination reaction. In PVC, a chlorine atom occupies 25 percent of the bonding sites on the backbone, while the remaining sites are filled by hydrogen. CPVC differs from PVC in that approximately 40 percent of the bonding sites on the backbone are filled with strategically placed chlorine atoms, while the remaining 60 percent of available sites are filled with hydrogen.
The chlorine atoms surrounding the carbon backbone of CPVC are large atoms, which protect the chain from attack. Access to the CPVC carbon chain is restricted by the chlorine on the molecule. It is the additional chlorine that provides CPVC with its superior temperature and chemical resistance.
CPVC | PVC
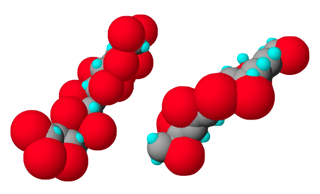
Diagram of CPVC (left) at a molecular level compared to PVC (right). The red spheres represent chlorine elements.
Industry-Accepted Standards for Evaluating Chemical Resistance
In addition to nearly 50 years of proven experience in the field, the chemical resistance capabilities of CPVC have been confirmed through work with numerous outside testing laboratories around the world.
Equally important is the fact that chemical resistance has been determined, and confirmed, using two widely accepted standards:
- ISO 22088
- ASTM D543.
ISO Evaluation
Part of ISO 22088 specifies methods for the determination of Environmental Stress Cracking (ESC) of thermoplastics when subjected to a constant tensile load in the presence of chemical agents.
Under this standard, materials are fully immersed in the chemical agent. If the chemical is highly viscous at the test temperature, the specimen is covered by a coating that is at least 2mm thick. During immersion, a constant tensile load is applied parallel to the longitudinal axis. In general, the maximum amount of stress to be applied is determined as being that which produces an elongation of 2 percent after one hour.
The ISO test can be conducted using two different approaches.
- The first approach utilizes a series of tests that increase the amount of stress until the material sample ruptures.
- In the second approach, the goal is to identify the amount of time it takes to rupture the material sample when subjected to a single stress load.
In either case, the test is run multiple times to ensure accurate results.
ASTM Evaluation
ASTM International has also developed standard practices for evaluating the resistance of plastics to chemical reagents. Standard D543 covers all plastic materials and provides specific guidelines as to how to administer the testing. This includes requirements on:
- Preparing the samples
- Conducting the actual tests
- Reporting the results
Similar to the ISO testing standard, there are two approaches that can be taken under ASTM D543.
- In the Immersion Test, the sample is totally immersed for a minimum of seven days. At the completion of the test, the sample is re-weighed and re-measured and compared
against its weight and measurements prior to immersion. The pipe surface is also observed for possible changes in texture, discoloration, swelling, clouding, tackiness, bubbling or cracking. - In the Mechanical Test, the sample is also exposed to the chemical reagent for a minimum time period, but during exposure, an external stress is applied (similar to ISO testing).
Even though both internationally accepted standards have proven reliable in their ability to determine a product’s chemical resistance, many pipe manufacturers still recommend that a test be conducted to replicate a plant’s process conditions. This will help to validate the expected reliability of the pipe in the presence of a specific chemical.
Suitable Chemical Applications
One of the most attractive features of CPVC piping is its resistance to corrosive chemicals–the same chemicals that can degrade and reduce the service life of many metals. In industrial applications, this corrosion can potentially cause expensive maintenance, disruption of operations and property-damaging leaks in these systems.
Many of the same chemicals that cause the greatest damage to metal piping, including acids, caustics salts, can be handled well by a properly installed CPVC piping system.
In addition, CPVC piping is not pH limited. It can accommodate wide pH swings in the fluids it transports.
As a result of its superior corrosion resistance, CPVC is proven acceptable for use with many different chemicals used in a wide array of industrial operations.
Industries that have already successfully installed CPVC piping systems include:
- Chemical processing
- Chlor-alkali
- Food and beverage
- Metal finishing (chrome plating)
- Mineral processing
- Power generation
- Pulp and paper
- Semiconductor
- Wastewater treatment
The Metal Finishing Industry
In the metal finishing/plating industry, there are numerous reasons why CPVC has proven to be a viable alternative to metal. The primary reason being the use of strong acids, including sulfuric acid for chrome and nickel stripping as well as nickel sulfate and nickel chloride.
In addition, the industry utilizes a many high-pH caustics. These corrosive chemicals, especially when used in high-temperature environments, can quickly create major corrosion problems. CPVC not only offers the necessary chemical- and temperature- resistance, but it also possesses the required impact and tensile strength.
The Chlor-Alkali Industry
Similarly, the chlor-alkali industry frequently transports a number of highly corrosive chemicals, including sulfuric acid, sodium hydroxide (caustic soda); cell liquor (brine, sodium hydroxide); sodium chloride (brine); sodium hypochlorite; hydrochloric acid; and demineralized/deionized water.
All of these have proven to be compatible with CPVC for use in steam condensate/evaporator systems, chlorine drying towers, and chlorine headers/manifolds, to name just a few applications.
The Wastewater Treatment Industry
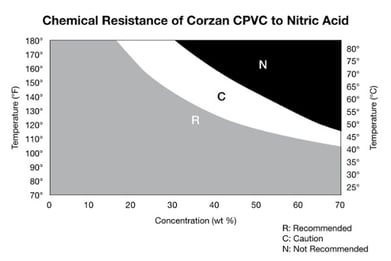
In wastewater treatment applications, harsh chemicals are a problem for many materials but can be handled easily and safely with CPVC piping. So, whether a plant is using acids such as sulfuric, hydrochloric, nitric or phosphoric acid, or bases including caustic (NaOH), magnesium hydroxide and calcium hydroxide (lime), CPVC has proven to be compatible.
It can even handle those chemicals that are used in conjunction with today’s treatment technologies, including ferric chloride, sodium hydroxide and sodium hypochlorite.

The Pulp and Paper Industry
In the pulp and paper industry, CPVC piping is ideal for use with pulp or bleaching operations, as well as for weak black liquor and green liquor in various storage and unit operations.
The Food and Beverage Industry
Since most food and beverage plants require meticulous cleaning to meet strict health standards, process equipment is frequently cleaned using harsh chemicals or cleaning agents at high temperatures. These conditions can corrode most metals and make many plastics unsuitable, due to the extreme temperatures. But CPVC possesses both the corrosion and temperature resistance needed to perform reliably and cost effectively.

Determining Chemical Compatibility Requires Many Considerations
Refer to Corzan® Industrial Systems’ Chemical Resistance Table for a full listing of chemicals that are both recommended and not recommended for use with CPVC piping.
Remember that chemical compatibility can be affected by many factors, including temperature, pressure and design of the system. Some piping manufacturers offer technical assistance and will actually test specific chemical formulations to determine chemical compatibility.
It is always recommended to work with the specific pipe and fitting manufacturer to determine material suitability.
It should further be noted that CPVC products are made with base resins having different molecular weights and chlorine content, as well as different compound additives. Therefore, it is always recommended to check with a specific pipe and fitting manufacturer before confirming actual chemical resistance capabilities and compatibility.
Caution Areas and Indications of Chemical Compatibility Problems
Chemical resistance issues can show up in a number of ways, with the most common problems being softening, degradation and cracking. If CPVC pipe has been incorrectly specified for a particular chemical application, or if it is unintentionally coming into contact with an incompatible material, several modes of failure can result.
Softening
Softening is a type of pipe failure characterized by a swollen and/or distorted appearance, which eventually causes a failure by ballooning and ductile rupture or by distortion of the system. Although there are several causes of softening, one primary cause is the absorption of solvents or plasticizers, either from the process fluid itself or from the external environment.
CPVC, generally, is not recommended for use with most solvents—soluble or insoluble–including ketones, ethers, furans, esters, alcohols and aromatics. Some water-soluble solvents may be suitable at low concentrations. However, water-insoluble solvents may be absorbed out of process fluid even if present at low concentrations.
Chemical contamination can arise from a variety of sources, including but not limited to:
- Oils can leak from pumps or other mechanical devices
- Plasticizers can leach from gaskets, hoses or tank linings
- Process fluids inside may not be as clean or as well defined as had been thought.
Solvents or plasticizers may also be absorbed from the external environment. Gasketing materials, caulks, rubber padding or lining materials may all contain plasticizers that can migrate into the rigid vinyl over time, leading to softening and eventual rupture under pressure. While the design engineer must be aware of these limitations, proper system design and specification of ancillary materials can prevent these types of compatibility issues.
Degradation
Degradation of CPVC piping materials occurs when the vinyl resin or other compounding ingredients in the material are altered or destroyed. It can manifest itself in many ways, including blackening and blistering of the material surface.
Degradation can be caused by prolonged exposure to conditions in excess of the recommended temperature, pressure and chemical concentration (TPC).
Often, degradation is due to exposure to chemicals capable of reacting with and destroying the base polymer of the compounding additives. Some of the most common problem chemicals include amines and ammonia.
As previously stated, CPVC offers superior resistance to many strong acids and caustics, and this is the reason it is often selected for use in a number of aggressive chemical environments, including industrial- strength bleach applications or metal pickling and plating baths.
However, no material is without its limitations. In excess of its recommended TPC limits, hot concentrated sulfuric acid may cause blackening and blistering, while hot concentrated nitric acid may cause whitening and surface etching.
A key difference between PVC and CPVC chemical resistance is in the area of ammonia and amine chemistries. In most cases, CPVC will outperform PVC in both its chemical and temperature resistance. However, PVC exhibits generally good resistance to ammonia and some amines, even at somewhat elevated temperatures. CPVC, on the other hand, has extremely poor resistance to ammonia or ammonium hydroxide, and limited resistance to most amines, even at ambient temperatures.
This is due to the extremely high reactivity of amines and chlorine, the higher availability of chlorine on the CPVC, and the lower carbon-chlorine bond strength of CPVC compared with PVC. Even at fairly low concentrations and temperatures, ammonia and many amines are capable of rapid dehydrochlorination of CPVC.
Environmental Stress Cracking
Environmental stress cracking, also referred to as ESC, is a mechanism by which an organic chemical (possibly a weak solvent or even a non-solvent) achieves an extremely localized weakening at the surface of the material, which permits propagation of a crack.
Environmental stress cracking generally presents itself as a crack with glossy fracture surfaces that occur in regions of high mechanical stresses. ESC is dependent on both the presence of the chemical and a significant level of mechanical stress. Therefore, it may occur in some installations or certain parts of a system, while the system performs well in other areas. Many problems can, as a result, be avoided by proper design and installation.
Potential ESC agents for CPVC include natural or synthetic ester oils, nonionic surfactants, alcohols and glycols.
Solvent Cement Joints: Reliable Even When Handling Harsh Chemicals
Chemical resistance capabilities are important to understand because they directly affect the reliability and long-term performance of the entire piping system. In addition to the chemical and mechanical properties of the pipe and fittings, however, it is also important to consider the integrity of the joint.
In many piping systems–metallic as well as non-metallic–the joint is often the most vulnerable point in the system, the most likely to fail, and the point at which most leaks will occur.
This is not the case with CPVC. In fact, over its nearly 50-year history in the field, CPVC piping joints have proven to be the strongest part of the system–stronger than either the pipe or fitting alone. This is due to the unique chemical bonding process of the solvent cement weld.
Although there are a number of methods for joining a CPVC piping system, in most operations CPVC pipe is joined with solvent cement.
CPVC and Solvent Cement
Despite the fact that some people liken solvent cement to adhesive glue, it is very different, inasmuch as the solvent cement creates a welded, permanent bond.
The solvents in the cement are designed to intentionally soften the surfaces of the pipe and fitting socket. Because the socket is tapered, the softened surfaces bond once they are pushed together. CPVC resin in the solvent cement fills in any gaps that might otherwise exist in the joint. As the joint cures, the solvents flash off.
As a result, the CPVC solvent cemented joint is permanent and reliable.

For exceptionally harsh chemical environments, special solvent cements are available and recommended. These have proven to be highly successful in even the most aggressive environments and compare favorably in mechanical performance to traditional solvent cements.
In fact, years ago, some plants experienced joint failures as a result of the CPVC solvent cement used. The fillers contained in the solvent material had dissolved from the effects of the harsh chemicals, and leaks occurred at the joint. These problems have since been remedied and proven reliable with newer formulations.
The designer should always consult with the pipe and fitting or solvent cement manufacturer to ensure the proper solvent cement is specified.
Conclusion
Chemical compatibility is critical when designing and installing an industrial piping system. Consideration must be given to the chemical makeup of all process fluids, as well as any other materials that are in contact with the CPVC pipe and fittings.
CPVC has proven through the years that it offers superior chemical resistance and a more reliable performance in a number of key chemical industries. it is easy to see why CPVC has become the material of choice for more and more industrial plants around the globe when combined with its other benefits, including:
- Lower material costs
- A faster, easier installation process
- Lightweight design
- Abrasion resistance
- Superior flame and smoke characteristics
- Weatherability
- Energy efficiency
But, like all other piping materials, it does have its weaknesses and should not be specified in applications for which it offers very little or no chemical resistance. This paper generically addressed the types of chemical environments in which CPVC performs well and those in which it does not.
Because there are so many factors that ultimately affect a pipe’s ability to perform safely, reliably, and cost-effectively, it is important to thoroughly research the material and work directly with the pipe and fitting manufacturer to ensure compatibility and a high-performance system.
To speak with a Corzan engineering and product specialist, contact us.
Michelle Knight is an R&D Scientist for Corzan Industrial Piping Systems with Lubrizol Advanced Materials, Inc. She holds a BSChE and a BSChem from Purdue University. Knight has more than 25 years of experience in materials testing and failure analysis.
Would You Like a PDF Version of This Information?
To receive this information in a PDF guide, click the button below.




