What is Deinozed Water and Which Piping Materials Convey it Effectively?
Demineralized or deionized water is used for many lab reactions, laboratory equipment washing, industrial processing applications and more. This water has been purified of ions, minerals, bacteria and other organics that may have been present.
Any of those contaminates can alter chemical reactions, cause scaling and corrosion for piping systems, and create a number of unique problems for specific applications. Once demineralized and deionized, water can still harm piping systems if the wrong material is specified because pure water becomes more reactive.
Learn about the use cases, qualities and preferred piping systems of purified water to keep your flow free of contaminates and your piping system running longer.
What Is Deionized or Demineralized Water?
Demineralized or deionized water has been purified and neutralized. This includes removing positively charged ions (cations), like calcium and magnesium, or negatively charged ions (anions), such as chloride and sulfate.
Ideally, the end result is water completely free of any charged particles, giving the water a neutral pH, high resistivity and essentially no dissolved solids.
By using deionized water, users ensure any ions that appear in natural or tap water will not inadvertently interfere with the process. A few use cases:
- Laboratories—whether at a middle school or research institute—use deionized water for experiments and for washing equipment used for reactions.
- In the pharmaceutical sector, each ion in a drug must be controlled, meaning highly pure water is required prevent interference.
- For the industrial sector, deionized water is turned into steam to turn power plant boilers, as dissolved minerals would cause corrosion and scaling, harming efficiency and causing excess downtime. And for process chemistries, deionized water is used because it will not add unwanted contaminants or minerals to the reaction.
Each example may require a slightly different level of purified or deionized water.
Levels of Purity
The ASTM Standards for Laboratory Reagent Water (ASTM D1193-91) defines the four different types of pure water, as shown in the chart below.
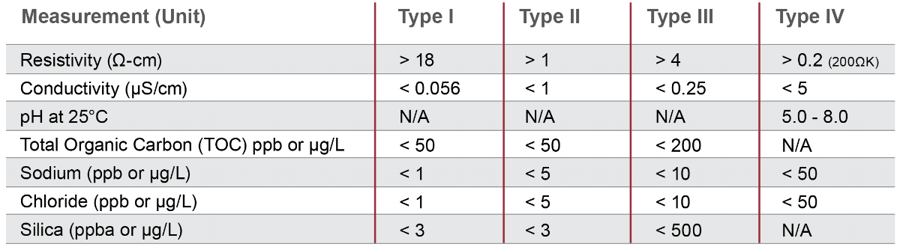 The ASTM standards are further subdivided into A, B and C that can be used in conjunction with the type I, II, III or IV water above when bacteria levels need to be controlled.
The ASTM standards are further subdivided into A, B and C that can be used in conjunction with the type I, II, III or IV water above when bacteria levels need to be controlled.
Electrical resistivity and electrical conductivity. This temperature-dependent quality quantifies the level to which water defies electricity. The more resistive the water is, the higher the purity. Greater resistivity indicates a lower level of ionic material.
pH. The pH is a measurement of water acidity or alkalinity on a scale of 1 to 14. Ultra-pure water has a neutral pH of 7.0, but is not required for Type I, II and III water because these grades of water do not have the necessary ions to be measured effectively. Resistivity and pH correlate, so resistivity can be used to identify the pH of water.
The following chart shows the relationship between electrical resistivity and deionized water.
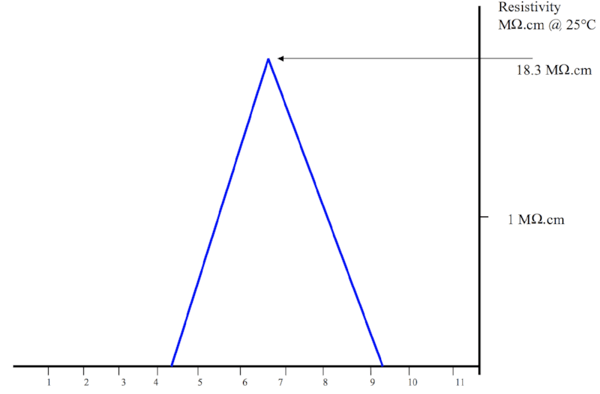
Image credit to the Pro-Analitika Kft
Total organic compound (TOC) in parts per million (ppm). Dissolved solids include any minerals, salts, metals, cations or anions dissolved in the water. Unsurprisingly, the less TOC in the water, the more pure it is considered. At room temperature, ASTM defines the following TOC for pure water:
- Type I: <50 ppm
- Type II: <50 ppm
- Type III: <200 ppm
For comparison, fresh water may contain up to 1,000 ppm TOC, and seawater may have 30,000-40,000 ppm TOC.
How Well Do Different Piping Materials Stand Up to Deionized Water?
Choosing the wrong piping material can have detrimental effects in different applications. Give strong consideration to piping material selection or risk it ruining chemical reactions or experiencing costly corrosion.
Key Considerations for Piping Material Selection
When determining which material to use to convey deionized water, three main performance factors should be considered:
- Leachability of the material.
- Reactivity of the material to the water it is conveying.
- Ability of the system to be used in a return air plenum.
If a material can be verified effective by those three issues, consider cost, installation and other non-application specific characteristics.
Plastics and Deionized Water
In general, plastics are known for their corrosion resistance and relative cost. A few options with various pros and cons are as follows:
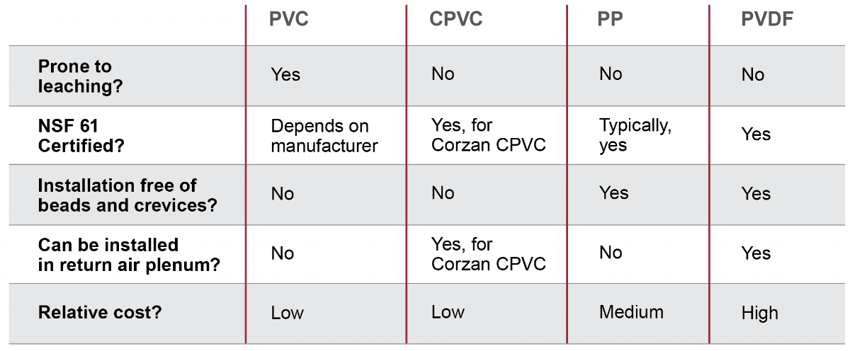
- Chlorinated polyvinyl chloride (CPVC). When conveying any type of water, CPVC will not corrode or show signs of wearing. It is resistant to the reactivity of the water, not prone to leaching and plenum rated to be installed in return air plenums; Corzan® CPVC fully complies with NSF 61, certifying its non-leaching properties and safety for use with potable water.
- Polyvinyl chloride (PVC). A commodity material, PVC is a cost-effective option at low purity levels, but is prone to leaching when pure water standards must be maintained. PVC can also be a fire hazard in return air plenums.
- Polypropylene (PP). An advantage over other materials is that PP offers installation methods that make pipe runs free of beads and crevices for ultra-pure applications, but that installation method requires special fusion equipment. Additionally, PP is not usable in return air plenums due to flammability concerns.
- Polyvinylidene fluoride (PVDF). As with polypropylene, PVDF can also be installed with bead and crevice-free connections with special training and fusion equipment. It also contains additives to ensure non-leaching properties and is plenum rated for return air plenums. The downside to PVDF is its cost, which is typically four or five times beyond CPVC’s cost.
Piping Materials and Purified Water Comparison Chart
Metals and Deionized Water
In many applications, metal is the material of choice due to its familiarity. Yet, in applications where purity is a priority, some metals are prone to corrosion and leaching.
- Copper. Copper is an example of a metal that should not be considered for deionized water applications—or any water applications. If the fluid’s pH ever falls below 6.5 (which can even occur to purified water), corrosion may occur and copper molecules can leach into the supply.
- Stainless steel. Stainless steel is the most common option due to historical use and because it is not prone to corrosion or leaching when used with deionized or pure water. At elevated levels of purity, stainless steel may leach over time and can corrode in case the water’s pH ever becomes too acidic or alkaline.
In laboratories and other purity-dependent processes, plastic piping should be strongly considered for its corrosion resistance, non-leaching properties, easy installation and related overall lifecycle costs.