Comparing Vessel and Piping Materials for Demineralization Ion Exchange Systems
In power generation boiler systems, water purity is critical, as the slightest contaminant can lead to deposits, corrosion and scaling on the turbine blades or tubing, reducing efficiency and limiting the life of the system.
Boiler water comes from natural bodies, which contain many impurities, including dissolved gases (i.e. oxygen and carbon dioxide) and minerals (e.g. calcium and magnesium) that must be removed from the fluid.
To remove the mineral impurities, the feed water goes through a demineralization process, which removes ions that can be detrimental to the system. In this post, our product and engineering team breaks down how demineralization is achieved through ion exchange, and offers advice on the vessel, container and piping materials best suited for this process.
How Ion Exchange Achieves Demineralization
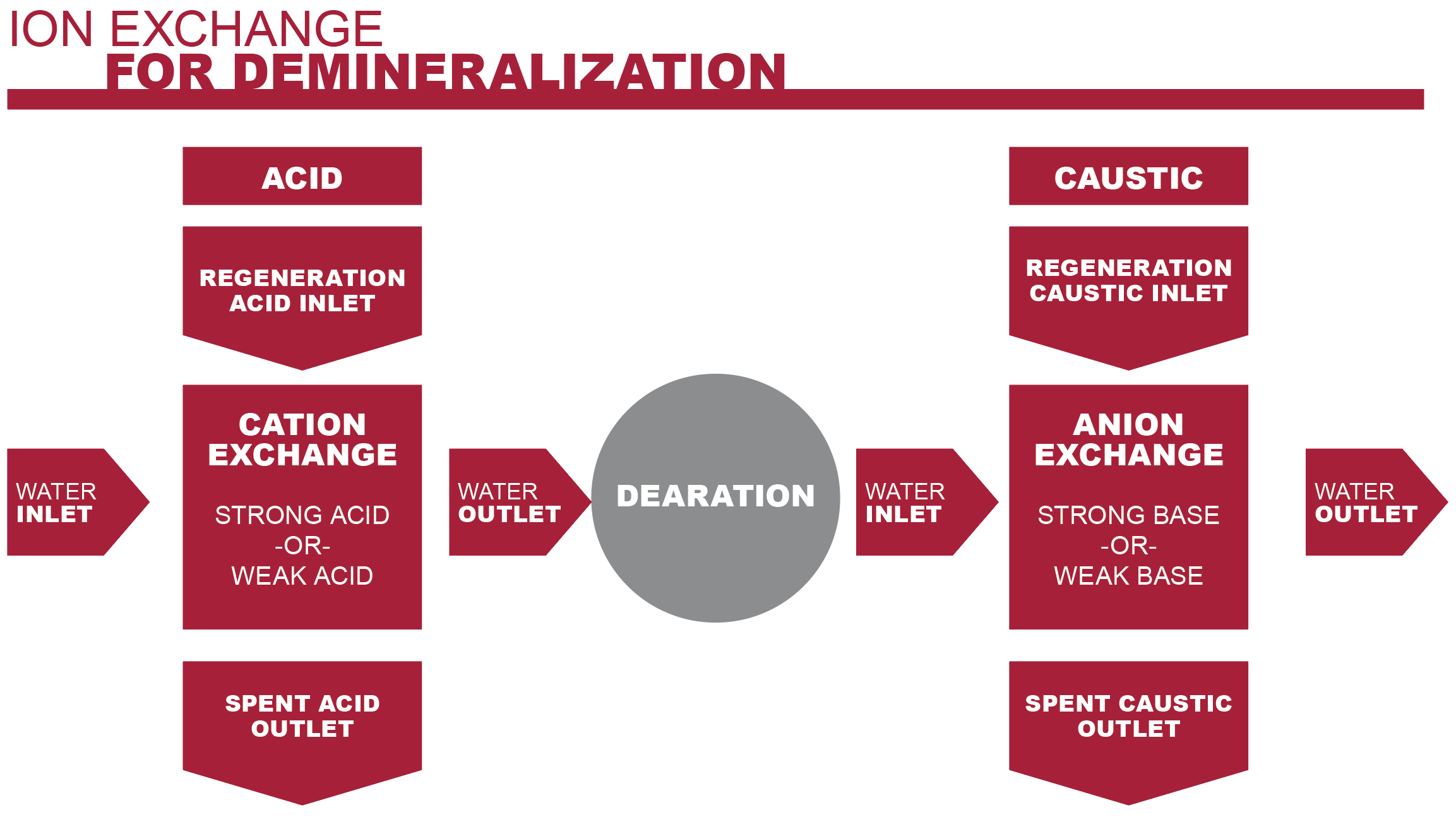
Ion exchange systems are designed to remove calcium and magnesium ions from the feed water. The process includes two separate exchanges: a cation exchange and anion exchange.
1. Cation Exchange
Processed water enters a cation exchange vessel first. The incoming water contains dissolved inorganic salts, such as sodium chloride, magnesium chloride and calcium chloride. Once dissolved, these salts disassociate, leaving free calcium and magnesium ions in the water.
The cation exchange vessel contains a strong acid cation resin. This resin is made up of charged particles or granules that include hydrogen ions. As the water moves through the resin bed, the resin granules will release a hydrogen ion in favor of a calcium or magnesium ion.
The result of this exchange is the calcium and magnesium ions are bound up, eliminating the chance of them depositing at some point in the system. Instead, what remains is the minerals’ corresponding acid.
2. Anion Exchange
Because boiler feed water must maintain a specific pH balance, the acids from the cation vessel must be neutralized.
The anion exchange vessel contains a bed of strong base anion resin. This base resin neutralizes the acids as the water flows through, balancing the water’s pH level.
The demineralized, pH balanced water is now free to flow into the next step of the boiler system without the possibility of scaling the boiler tubes.
Regenerating Ion Demineralization Resin Beds
Over time, these resin become less effective at removing either mineral ions or neutralizing the fluid’s acidity. Most boiler operators monitor the fluid flow for indicators that the resin has reached the end of its life:
- Cation Exchange: Free mineral acidity of a fluid drops sharply.
- Anion Exchange: Effluent silica levels increase sharply, and conductivity levels decrease momentarily and then rise sharply as well.
When either of these instances occur, the system is programmed to begin the regeneration process.
Regeneration of Cation Exchange Resin
While any mineral acid (e.g. hydrochloric acid) will do, sulfuric acid is typically applied to the resin because it is the least expensive. The sulfuric acid is applied to the resin in a low concentration (between 2% and 8%). Too much sulfuric acid can irreversibly foul the resin.
The sulfuric acid restores the exchange sites to their hydrogen form, allowing the ion exchanges to take place again.
Regeneration of Anion Exchange Resin
To regenerate the anion exchange resin, a caustic solution of 4% sodium hydroxide is applied. This solution is typically applied at a temperature of 120°F (48.9°C) to improve process efficiency.
Both of these regeneration processes place specific demands on container, piping and vessel materials, as does the process of recovering spent acid and caustic solutions.
Demineralization Ion Exchange Material Options
The temperature, pressure and range of solutions used in the demineralization process place unique demands on the materials used for containers, piping, fittings, valves and vessels. The proper material choice can prolong the life and efficiency of the system, and deliver measurable savings.
Metals: Low concentrated acids and caustic solutions inherently corrode metals. In addition, calcium and magnesium ions can attach to the metal walls, leading to scaling. Metals are not recommended for ion exchange.
Rubber-Lined Steel: Rubber-lined steel is an effective choice for limiting corrosion and scale concerns. The rubber is inert to a range of solutions, and the steel provides the necessary strength to satisfy heat and pressure demands. However, rubber-lined steel tends to be more costly than other material options.
PVC: Polyvinyl Chloride (PVC) is inert to many of the solutions used throughout the demineralization process, but often can’t satisfy pressure or elevated temperature conditions, specifically during anion exchange resin regeneration.
CPVC: Chlorinated Polyvinyl Chloride (CPVC), specifically Corzan® SCH 80 CPVC pipe, provides an ideal solution. It is inherently inert to acids, bases and salts eliminating corrosion concerns, and doesn’t attract the metal ions that cause scaling.
CPVC is also pressure rated beyond the temperature and pressure demands of most power generation feed water demineralization systems. Finally, it offers one of the lowest installed costs of any material from a piping perspective.
If you have any questions about material choice for your boiler feed water demineralization process, our team of product and engineering specialists are always available. Schedule a free consultation.
Can Corzan® CPVC Stand Up to the Extreme Chemicals in Your Plant?
View the Corzan Chemical Resistance Table for CPVC's compatibility with 400+ chemicals.