SAFER ALTERNATIVES TO CHLORINE GAS FOR WATER AND WASTEWATER TREATMENT DISINFECTION
In the 1930s, chlorine gas became commercially available and applications around the world began to take advantage of it as a disinfectant. Today, chlorine is the most widely used disinfectant in water and wastewater treatment processes.
Growing concern over the past few decades about the health and safety of chlorine, especially in its gaseous state, has many plants considering alternatives.
Why Disinfection Is Needed
The wastewater treatment industry, which includes nearly 15,000 plants in the United States alone, involves the treatment of wastewater from agriculture, sewage and industrial plants.
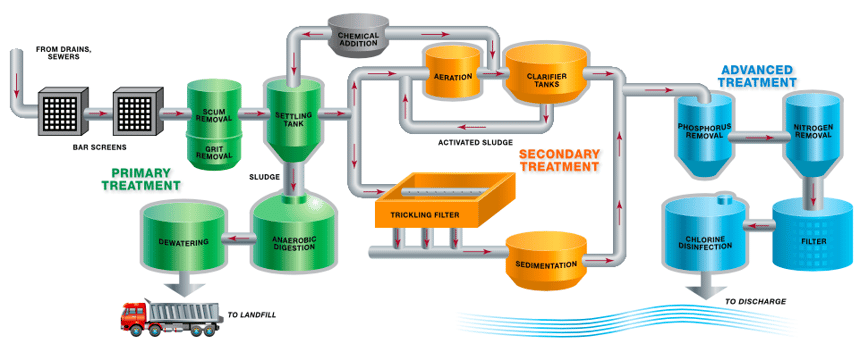
The treatment process typically involves three stages—primary, secondary and advanced or tertiary treatment.
Following primary treatment and secondary treatment, 85% of all organic matter should be removed. During advanced treatment, the wastewater is disinfected to reduce (or remove) pathogens that can be harmful to humans, animals and plants.
Whether the treated water is eventually used for drinking or is discharged into the environment, some level of disinfection is often required.
About Chlorine Gas
Chlorine gas is greenish-yellow, with a density about double that of air. Its tremendous use as a disinfectant has made it the disinfection method of two-thirds of all drinking water facilities, but some potential disadvantages are causing water and wastewater plants to consider alternatives.
Chlorine Gas Pros
Chlorine in general is one of the most effective disinfectants, and is known to deactivate most pathogenic microorganisms. Specifically, chlorine gas is involved in wastewater treatment because it is cheaper and occupies less space for storing when compared to chlorine solutions.
Chlorine Gas Cons
Along with the positives of chlorine as, there are a few related health and safety concerns when it comes to wastewater treatment facilities:
- It is a toxic respiratory irritant. Exposure to 4 ppm for more than an hour can cause significant respiratory affects.
- Certain reactions cause chlorine gas explosions, including acetylene, ammonia, hydrogen and fluorine.
- Wet chlorine is extremely corrosive.
Due to the potential dangers of chlorine gas, federal regulations limit the amount a single location can store at a time, resulting in special storage and handling considerations.
With the known hazards of chlorine gas—especially when stored on site in high volumes—some water and wastewater treatment are considering disinfection alternatives or other chlorine gas storage techniques.
Alternatives to High Volume Storage of Chlorine Gas
To utilize the strengths of chlorine gas for disinfection, without having to store it in large volumes, on-site generation is a viable alternative.
Emerging technological developments are making on-site generation of chlorine gas, hypochlorite and other disinfectants increasingly accessible, efficient and cost-effective.
On-Site Chlorine Gas Generation (OSCG)
In on-site chlorine gas generation (OSCG), chlorine gas is generated through the electrolysis of a salt-water solution. The process involves a sodium chloride and water solution and applies electricity. The reaction produces chlorine gas with sodium hydroxide and hydrogen gas byproducts.
Rather than storing massive quantities of chlorine gas on-site, OSCG gives a plant on-demand access to this highly effective disinfectant without the same safety concerns.
On-Site Hypochlorite (OSSG) Generation
The most efficient and cost-effective way to use hypochlorite—compared to bulk commercial transport and storage—is for the plant to generate it onsite.
To do this, an oxidation reaction is carried out between sodium chloride and water, forming sodium hypochlorite.
Handling On-Site Generation Systems
While on-site generation is often safer, more efficient and more cost-effective than alternatives, plants must be confident in the reliability of their on-site generation system.
Chemicals involved in or formed during on-site generation may include chlorine gas, hypochlorite, caustics, hydrogen chloride (HCl), and brine—all some of the most corrosive chemicals faced in water and wastewater treatment plants. The specified piping and tank materials must withstand a wide pH range, corrosion and other compatibility concerns.
CPVC is a technology ideally suited for the on-site generation process. To see how Corzan® CPVC stands up to more than 400 chemicals, check out the Corzan CPVC Chemical Resistance Table.
Alternatives to Chlorine Gas
If on-site generation is not an option for your plant, consider other alternatives for disinfection in place of chlorine gas.
Sodium Hypochlorite (NaClO)
Known more commonly as liquid bleach, this light yellow liquid is a chlorine derivative that is much safer than chlorine gas. Because it is produced and stored as a liquid, the potential danger of having high-pressure chlorine gas on site is eliminated. And, in this form, a lower concentration (5-15%) of chlorine is needed for water disinfection than with chlorine gas or calcium hypochlorite.
Approximately one gallon of industrial strength sodium hypochlorite (10-12% concentration) is required to replace one pound of chlorine gas.
When sodium hypochlorite (NaClO) is introduced to water, the chemical reaction forms hypochlorous acid and a hypochlorite ion. When those combine, free available chlorine (FAC) forms and acts as the disinfectant.
It is an especially practical alternative for use with low-hardness water. Mixing high-pH sodium hypochlorite with high-hardness waters can create calcium salts and promote scaling.
Though safer than chlorine gas, precautions should be taken with sodium hypochlorite to limit exposure. It can cause skin, stomach and throat irritation, depending on the extent or type of contact.
Stability of the solution is also an issue, as it is affected by heat, light, pH and metal contamination. Each factor promotes breakdown of the solution, reducing effectiveness and relative shelf life more quickly than alternatives.
Calcium Hypochlorite (Ca(ClO)2)
Manufactured from chlorine gas, calcium hypochlorite is typically available in a solid or dry form as white pellets or granules.
The concentrated chlorine in calcium hypochlorite is around 65%, much higher than sodium hypochlorite, resulting in higher costs.
While greater upfront costs are incurred, calcium hypochlorite offers lower handling costs than the two most common disinfectants.
Calcium hypochlorite maintains its stability and efficacy during storage better than sodium hypochlorite, but can be a hazard if subjected to heat or stored in or near an easily oxidized organic material or in metal.
Bromine (Br)
Bromine is a heavy red-brown liquid that is a viable alternative to chlorine for water disinfection when water is released into the environment.
Because ammonia is present in sewage water, bromamines are produced through injection of bromine and are even more effective than chloramines.
The downside to bromine is its relative cost in most situations. Usage is currently limited mostly to navy ships and oil drilling platforms, as it can be extracted from seawater. It also is not recommended for drinking water because it has harmful byproducts and it gives water a poor taste.
Chlorine Dioxide (ClO2)
Chlorine dioxide is a green-yellowish gas with similar advantages and disadvantages to chlorine gas. It is an effective disinfectant, but is extremely potent and can be hazardous if not properly handled.
If chlorine dioxide is used for disinfection in a plant, whether generated on-site or off-site, CPVC is an excellent material for handling the chemical. Because chlorine dioxide is unstable at most temperatures, pipes and vessels must securely withstand the oxygen given off as the material degrades. Polyolefins and fluorinated polyolefins, for example, are susceptible to oxygen permeation.
Corzan CPVC has the chemical resistant properties necessary to handle chlorine dioxide and its byproducts.
Transporting and Storing Corrosive and Oxidizing Chemicals
Each of the available disinfectants for water and wastewater treatment can cause corrosion and damage to certain materials. CPVC piping and tanks are ideal for storing and conveying many disinfectants and other chemicals. Learn how CPVC is used effectively throughout wastewater treatment plants by downloading the guide, CPVC Use in Wastewater Treatment Plants.


